

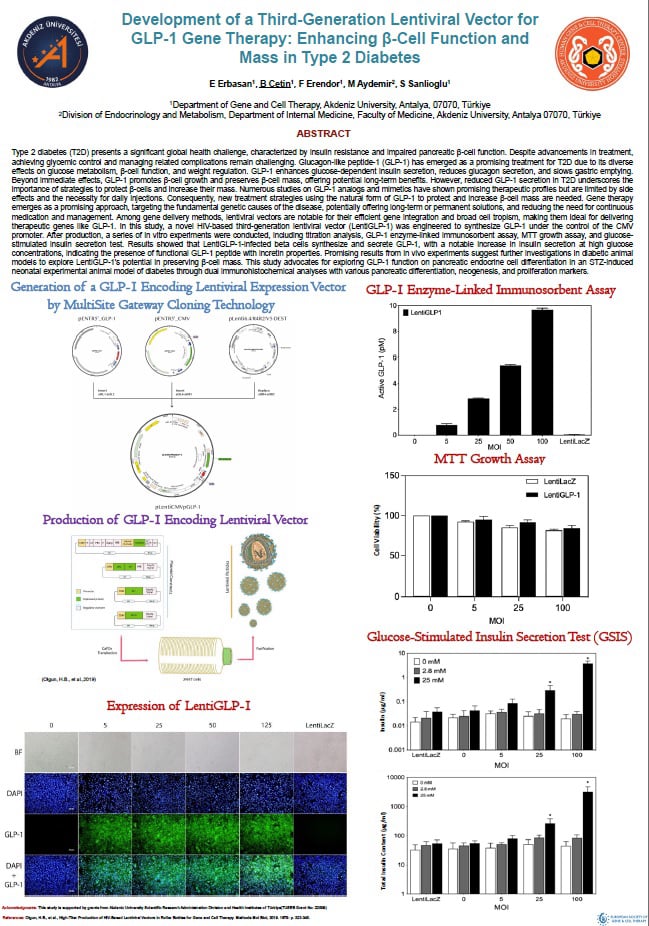
Type 2 diabetes (T2D) presents a significant global health challenge, characterized by insulin resistance and impaired pancreatic β-cell function. Despite advancements in treatment, achieving glycemic control and managing related complications remain challenging. Glucagon-like peptide-1 (GLP-1) has emerged as a promising treatment for T2D due to its diverse effects on glucose metabolism, β-cell function, and weight regulation. GLP-1 enhances glucose-dependent insulin secretion, reduces glucagon secretion, and slows gastric emptying. Beyond immediate effects, GLP-1 promotes β-cell growth and preserves β-cell mass, offering potential long-term benefits. However, reduced GLP-1 secretion in T2D underscores the importance of strategies to protect β-cells and increase their mass. Numerous studies on GLP-1 analogs and mimetics have shown promising therapeutic profiles but are limited by side effects and the necessity for daily injections. Consequently, new treatment strategies using the natural form of GLP-1 to protect and increase β-cell mass are needed. Gene therapy emerges as a promising approach, targeting the fundamental genetic causes of the disease, potentially offering long-term or permanent solutions, and reducing the need for continuous medication and management. Among gene delivery methods, lentiviral vectors are notable for their efficient gene integration and broad cell tropism, making them ideal for delivering therapeutic genes like GLP-1. In this study, a novel HIV-based third-generation lentiviral vector (LentiGLP-1) was engineered to synthesize GLP-1 under the control of the CMV promoter. After production, a series of in vitro experiments were conducted, including titration analysis, GLP-1 enzyme-linked immunosorbent assay, MTT growth assay, and glucose-stimulated insulin secretion test. Results showed that LentiGLP-1-infected beta cells synthesize and secrete GLP-1, with a notable increase in insulin secretion at high glucose concentrations, indicating the presence of functional GLP-1 peptide with incretin properties. Promising results from in vivo experiments suggest further investigations in diabetic animal models to explore LentiGLP-1's potential in preserving β-cell mass. This study advocates for exploring GLP-1 function on pancreatic endocrine cell differentiation in an STZ-induced neonatal experimental animal model of diabetes through dual immunohistochemical analyses with various pancreatic differentiation, neogenesis, and proliferation markers.
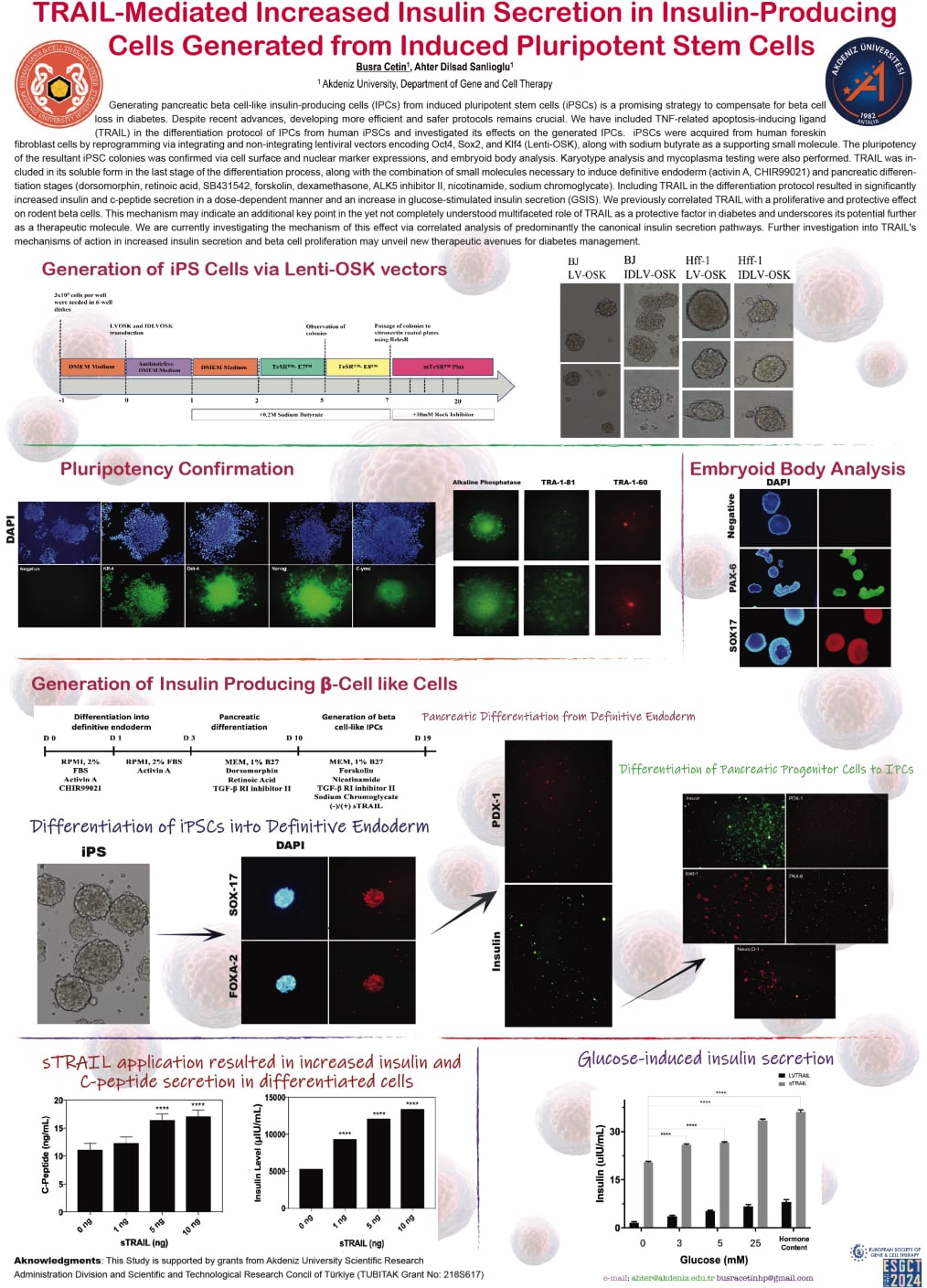
Generating pancreatic beta cell-like insulin-producing cells (IPCs) from induced pluripotent stem cells (iPSCs) is a promising strategy to compensate for beta cell loss in diabetes. Despite recent advances, developing more efficient and safer protocols remains crucial. We have included TNF-related apoptosis-inducing ligand (TRAIL) in the differentiation protocol of IPCs from human iPSCs and investigated its effects on the generated IPCs. iPSCs were acquired from human foreskin fibroblast cells by reprogramming via integrating and non-integrating lentiviral vectors encoding Oct4, Sox2, and Klf4 (Lenti-OSK), along with sodium butyrate as a supporting small molecule. The pluripotency of the resultant iPSC colonies was confirmed via cell surface and nuclear marker expressions, and embryoid body analysis. Karyotype analysis and mycoplasma testing were also performed. TRAIL was included in its soluble form in the last stage of the differentiation process, along with the combination of small molecules necessary to induce definitive endoderm (activin A, CHIR99021) and pancreatic differentiation stages (dorsomorphin, retinoic acid, SB431542, forskolin, dexamethasone, ALK5 inhibitor II, nicotinamide, sodium chromoglycate). Including TRAIL in the differentiation protocol resulted in significantly increased insulin and c-peptide secretion in a dose-dependent manner and an increase in glucose-stimulated insulin secretion (GSIS). We previously correlated TRAIL with a proliferative and protective effect on rodent beta cells. This mechanism may indicate an additional key point in the yet not completely understood multifaceted role of TRAIL as a protective factor in diabetes and underscores its potential further as a therapeutic molecule. We are currently investigating the mechanism of this effect via correlated analysis of predominantly the canonical insulin secretion pathways. Further investigation into TRAIL's mechanisms of action in increased insulin secretion and beta cell proliferation may unveil new therapeutic avenues for diabetes management.
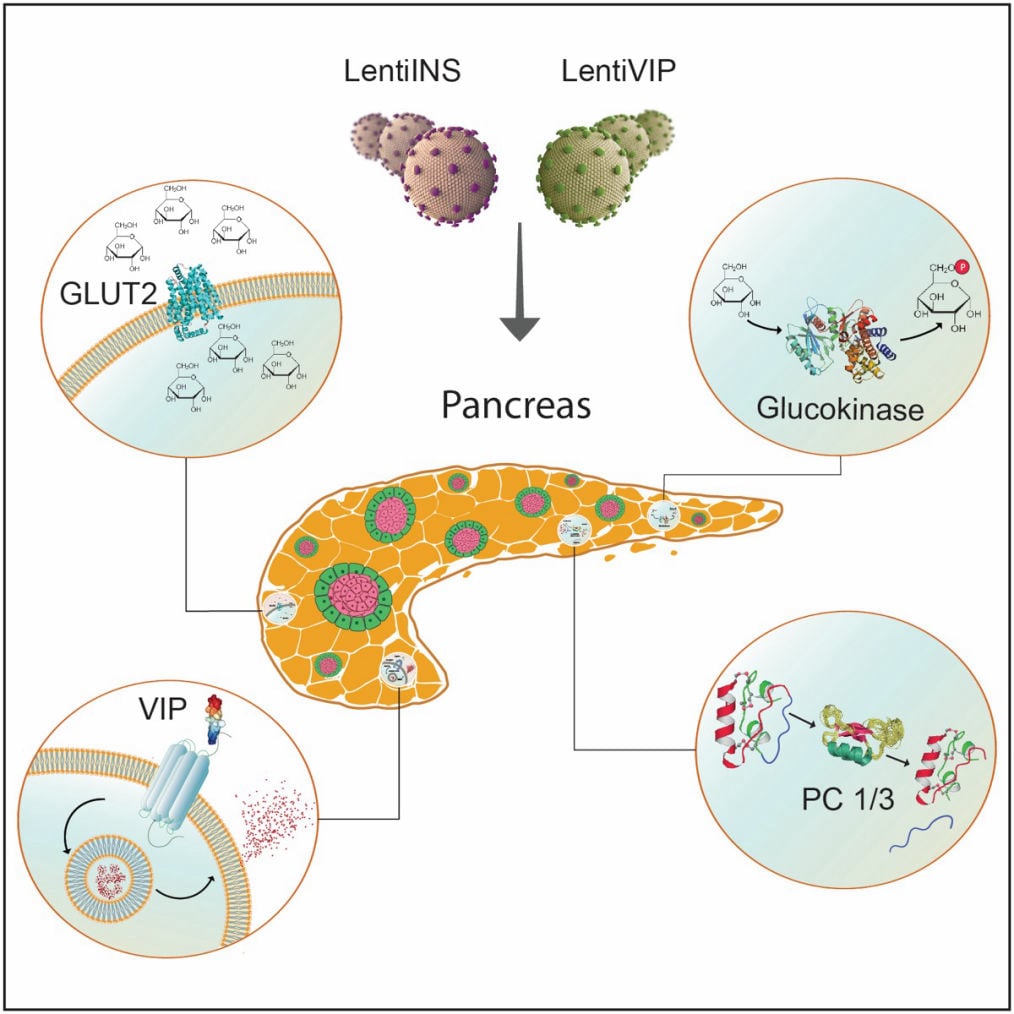
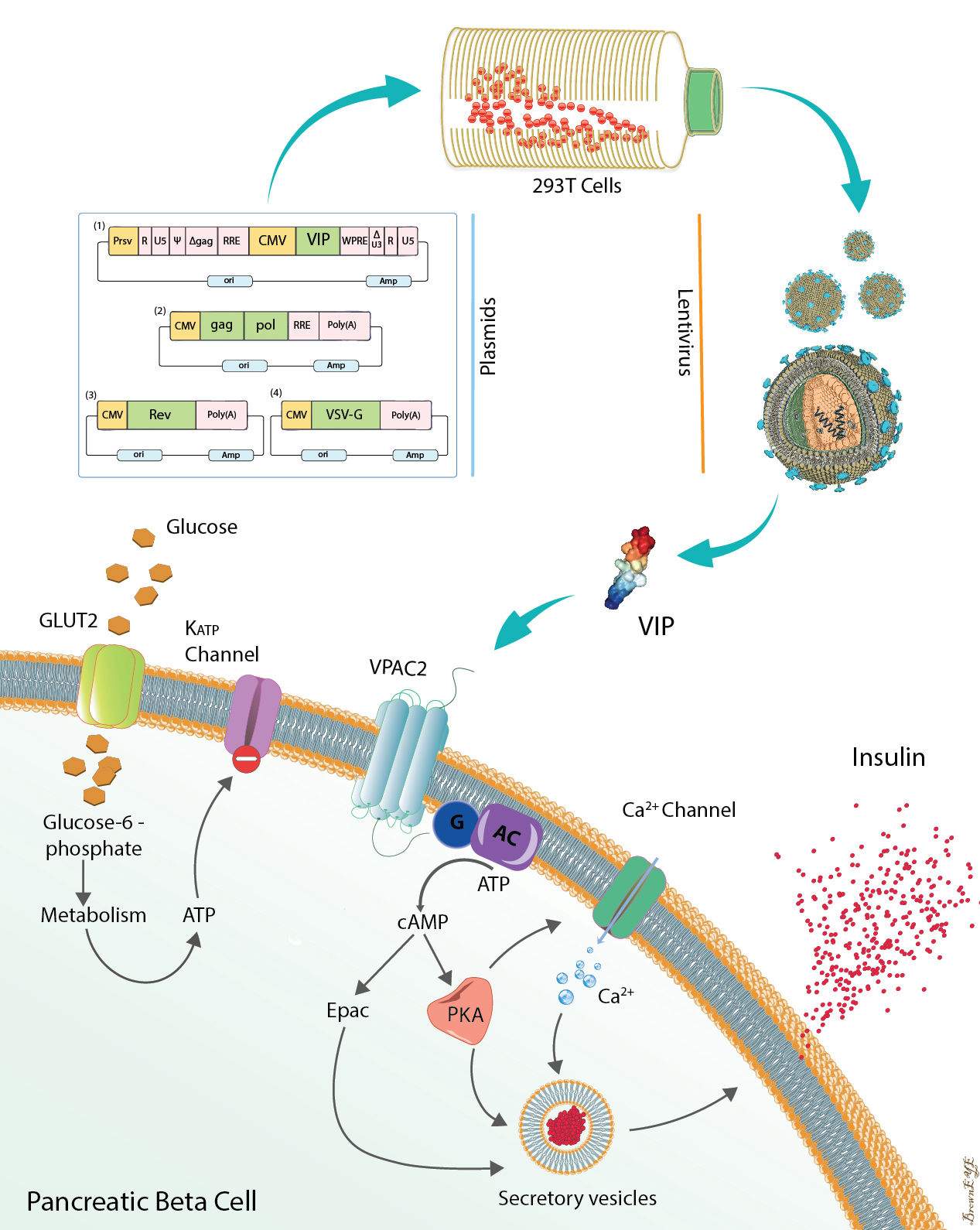
Functional consequences of pancreatic beta cell-specific insulin gene expression (LentiINS) complemented with anti-inflammatory gene delivery (LentiVIP). The unique properties of pancreatic beta cells are outlined in the figure. First, pancreatic beta cells possess a regulated secretory pathway allowing insulin storage. Glucose transporter 2 (GLUT2) and glucokinase (GK) function as cellular glucose radars. Prohormone convertase (PC1/3 and PC2) changes proinsulin to insulin. LentiINS gene delivery causes pancreatic beta-cell restricted glucose-regulated insulin gene expression which is augmented by LentiVIP. Concurrent use of LentiINS and LentiVIP stimulates pancreatic beta cell regeneration and proliferation in STZ induced diabetic rats. The anti-inflammatory properties of LentiVIP are not depicted for clarity.
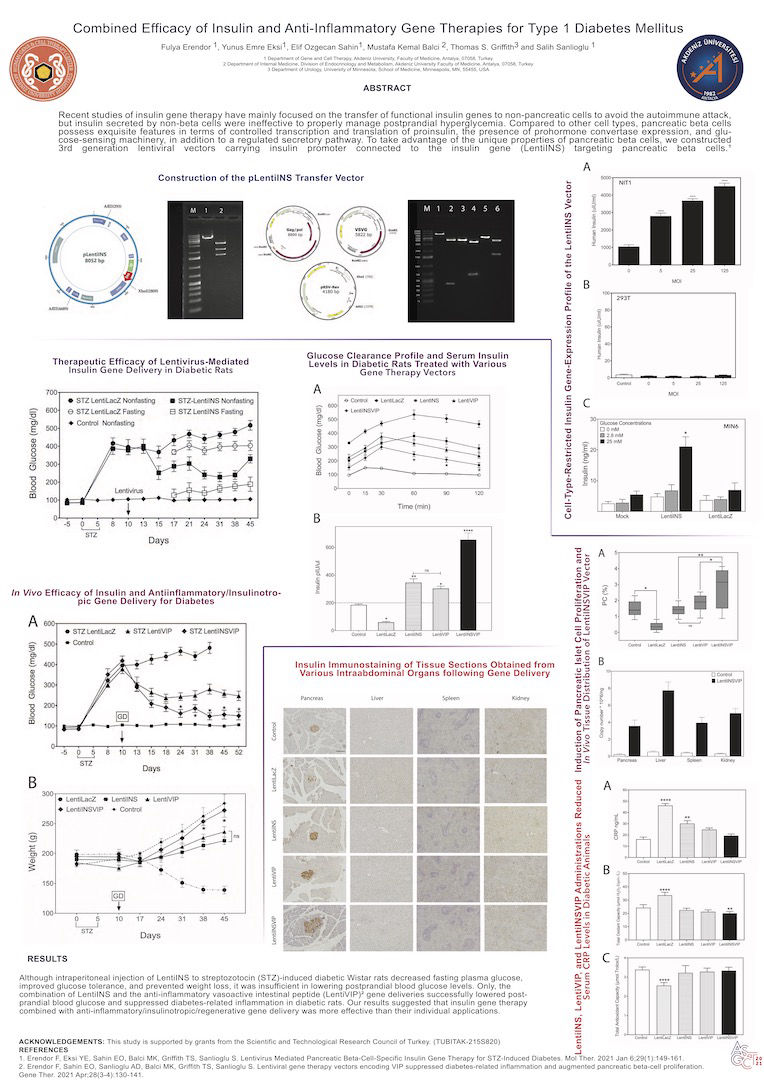
Recent studies of insulin gene therapy have mainly focused on the transfer of functional insulin genes to non-pancreatic cells to avoid the autoimmune attack, but insulin secreted by non-beta cells were ineffective to properly manage postprandial hyperglycemia. Compared to other cell types, pancreatic beta cells possess exquisite features in terms of controlled transcription and translation of proinsulin, the presence of prohormone convertase expression, and glucose-sensing machinery, in addition to a regulated secretory pathway. To take advantage of the unique properties of pancreatic beta cells, we constructed 3rd generation lentiviral vectors carrying insulin promoter connected to the insulin gene (LentiINS) targeting pancreatic beta cells.
Although intraperitoneal injection of LentiINS to streptozotocin (STZ)-induced diabetic Wistar rats decreased fasting plasma glucose,
improved glucose tolerance, and prevented weight loss, it was insufficient in lowering postprandial blood glucose levels. Only, the
combination of LentiINS and the anti-inflammatory vasoactive intestinal peptide (LentiVIP) gene deliveries successfully lowered post-prandial blood glucose and suppressed diabetes-related inflammation in diabetic rats. Our results suggested that insulin gene therapy
combined with anti-inflammatory/insulinotropic/regenerative gene delivery was more effective than their individual applications.
Combined Efficacy of Insulin and Anti-Inflammatory Gene Therapies for Type 1 Diabetes Mellitus. Fulya Erendor, Yunus Emre Eksi, Elif Ozgecan Sahin, Mustafa Kemal Balci, Thomas S. Griffith and Salih Sanlioglu
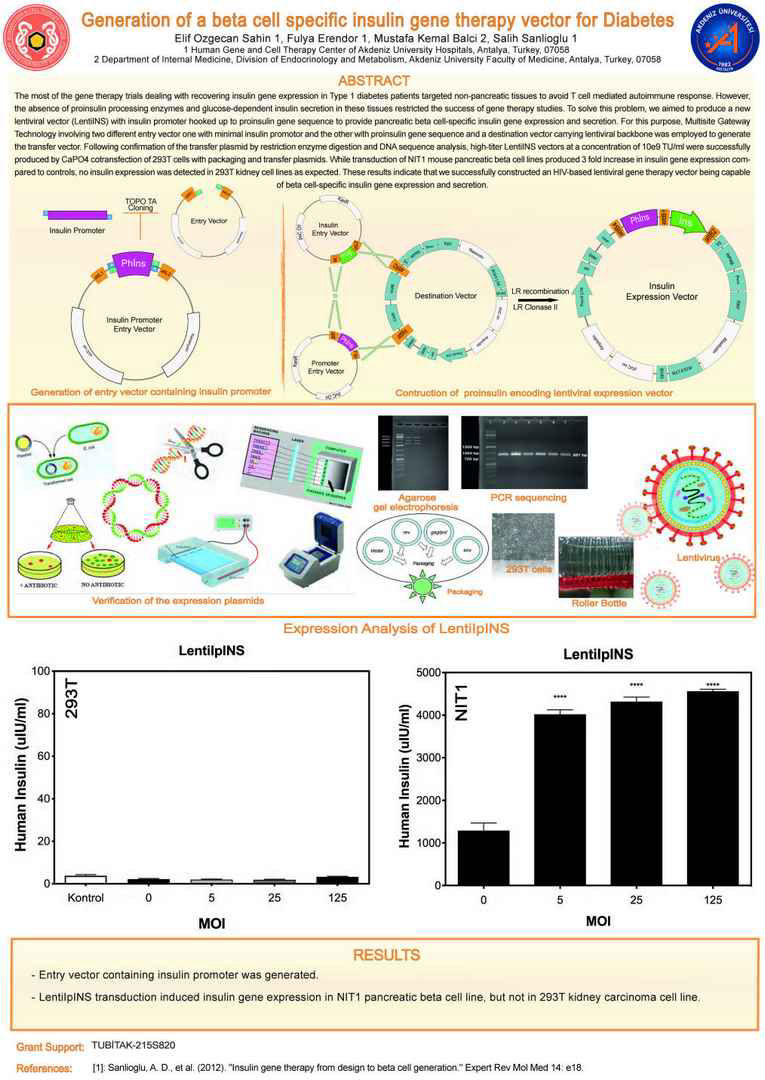
The most of the gene therapy trials dealing with recovering insulin gene expression in Type 1 diabetes patients targeted non-pancreatic tissues to avoid T cell mediated autoimmune response. However, the absence of proinsulin processing enzymes and glucose-dependent insulin secretion in these tissues restricted the success of gene therapy studies. To solve this problem, we aimed to produce a new
lentiviral vector (LentiINS) with insulin promoter hooked up to proinsulin gene sequence to provide pancreatic beta cell-specific insulin gene expression and secretion. For this purpose, Multisite Gateway Technology involving two different entry vector one with minimal insulin promotor and the other with proinsulin gene sequence and a destination vector carrying lentiviral backbone was employed to generate
the transfer vector. Following confirmation of the transfer plasmid by restriction enzyme digestion and DNA sequence analysis, high-titer LentiINS vectors at a concentration of 10e9 TU/ml were successfully
produced by CaPO4 cotransfection of 293T cells with packaging and transfer plasmids. While transduction of NIT1 mouse pancreatic beta cell lines produced 3 fold increase in insulin gene expression compared
to controls, no insulin expression was detected in 293T kidney cell lines as expected. These results indicate that we successfully constructed an HIV-based lentiviral gene therapy vector being capable
of beta cell-specific insulin gene expression and secretion.
Generation of a beta cell specific insulin gene therapy vector for diabetes. Sahin EO, Erendor F, Balci MK and Sanlioglu S. ESGCT XXV Anniversary Congress. Human Gene Therapy. Volume:28 Issue:12 Pages:A110 Meeting Abstract: P341. Berlin, Germany OCT 17-20, 2017
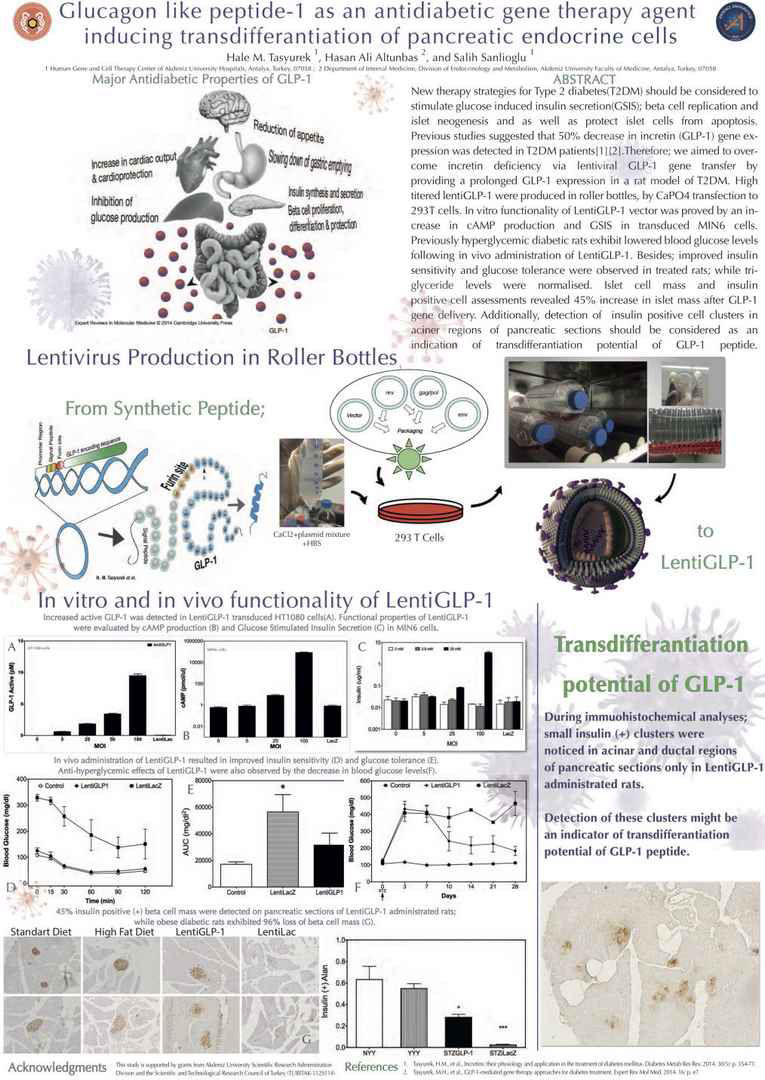
New therapy strategies for Type 2 diabetes(T2DM) should be
considered to stimulate glucose induced insulin secretion(GSIS);
beta cell replication and islet neogenesis and as well as protect
islet cells from apoptosis. Previous studies suggested that 50%
decrease in incretin (GLP-1) gene expression was detected in
T2DM patients[1][2]. Therefore; we aimed to overcome incretin
deficiency via lentiviral GLP-1 gene transfer by providing a
prolonged GLP-1 expression in a rat model of T2DM. High
titered lentiGLP-1 were produced in roller bottles, by CaPO4
transfection to 293T cells. In vitro functionality of LentiGLP-1
vector was proved by an increase in cAMP production and GSIS
in transduced MIN6 cells. Previously hyperglycemic diabetic
rats exhibit lowered blood glucose levels following in vivo administration
of LentiGLP-1. Besides; improved insulin sensitivity
and glucose tolerance were observed in treated rats; while
triglyceride levels were normalised. Islet cell mass and insulin
positive cell assessments revealed 45% increase in islet mass
after GLP-1 gene delivery. Additionally, detection of insulin
positive cell clusters in aciner regions of pancreatic sections
should be considered as an indication of transdifferantiation
potential of GLP-1 peptide. TUBITAK-112S114 .
Glucagon like peptide-1 as an antidiabetic gene therapy agent inducing transdifferantiation of pancreatic endocrine cells. Tasyurek H, Altunbas HA and Sanlioglu S. ESGCT XXV Anniversary Congress. Human Gene Therapy. Volume:28 Issue:12 Pages:A93 Meeting Abstract: P277. Berlin, Germany OCT 17-20, 2017
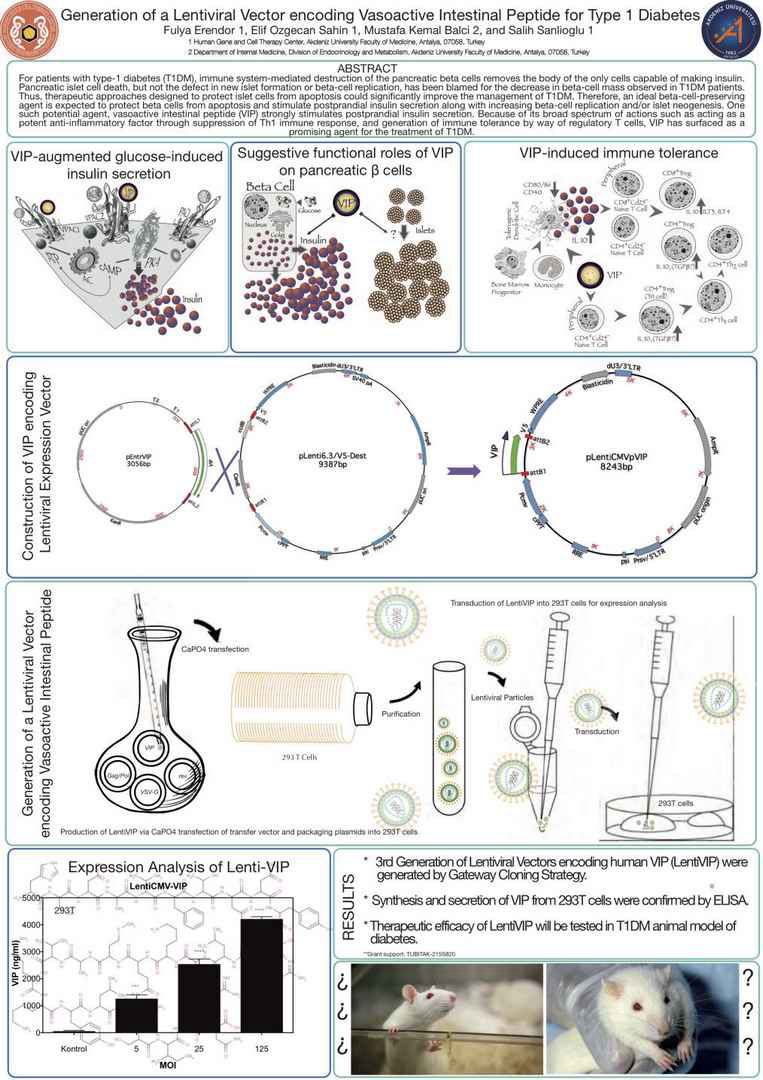
For patients with type-1 diabetes (T1DM), immune systemmediated destruction of the pancreatic beta cells removes the body of the only cells capable of making insulin. Pancreatic islet cell death, but not the defect in new islet formation or beta-cell
replication, has been blamed for the decrease in beta-cell mass
observed in T1DM patients. Thus, therapeutic approaches designed
to protect islet cells from apoptosis could significantly
improve the management of T1DM. Therefore, an ideal betacell-
preserving agent is expected to protect beta cells from apoptosis
and stimulate postprandial insulin secretion along with
increasing beta-cell replication and/or islet neogenesis. One such
potential agent, vasoactive intestinal peptide (VIP) strongly
stimulates postprandial insulin secretion. Because of its broad
spectrum of actions such as acting as a potent anti-inflammatory
factor through suppression of Th1 immune response, and generation
of immune tolerance by way of regulatory T cells, VIP
has surfaced as a promising agent for the treatment of T1DM.
Since VIP is a neuropeptide with insulinotropic and antiinflammatory
functions, 3rd generation lentiviral vectors encoding
human VIP (LentihVIP) were constructed using Gateway
cloning strategy. Restriction enzyme analysis and DNA sequencing
were utilized to confirm the cloning. The lentiviral
particle titers were quantified using QuickTiter HIV Lentivirus
Quantitation kit (Cell Biolabs). The expression was confirmed
by ELISA while functional status was revealed by glucose induced
insulin secretion test. Financial support: (TUBITAK-
215S820).
Generation of a lentiviral vector encoding vasoactive intestinal peptide for type 1 diabetes. Erendor F, Sahin EO, Balci MK and Sanlioglu S. ESGCT XXV Anniversary Congress. Human Gene Therapy. Volume:28 Issue:12 Pages:A59-A60 Meeting Abstract: P152. Berlin, Germany OCT 17-20, 2017
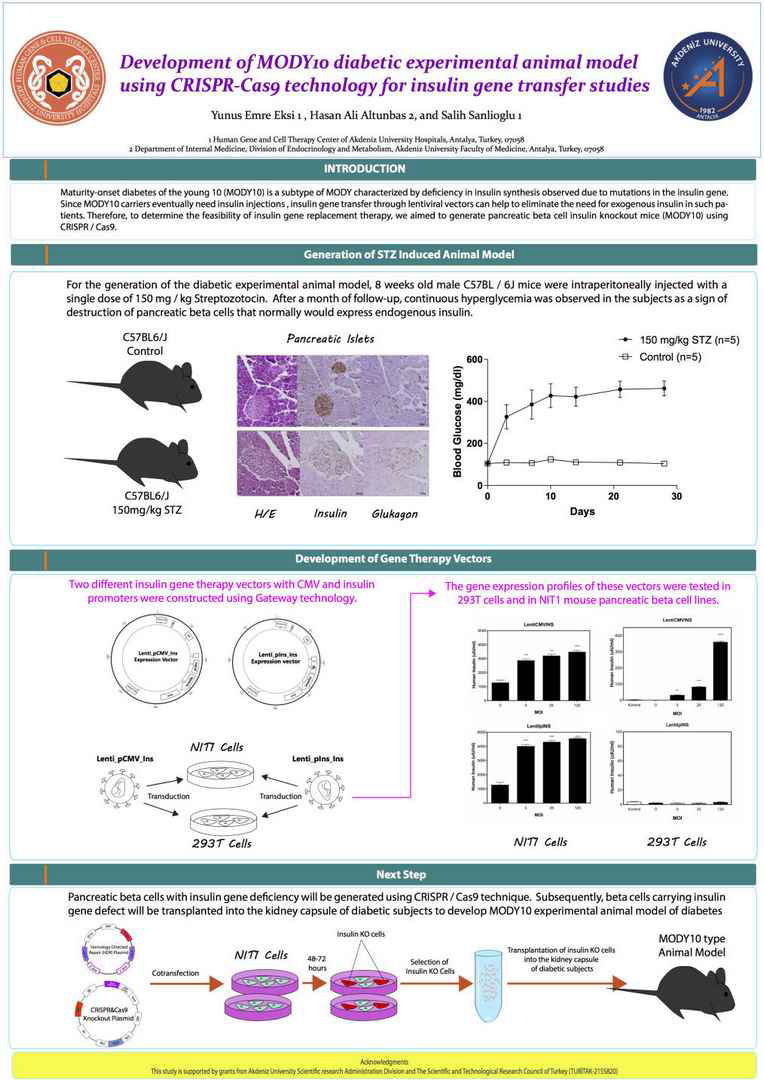
Maturity-onset diabetes of the young 10 (MODY10) is a
subtype of MODY characterized by deficiency in insulin synthesis
observed due to mutations in the insulin gene. Since
MODY10 carriers eventually need insulin injections, insulin
gene transfer through lentiviral vectors can help to eliminate the
need for exogenous insulin in such patients. Therefore, to determine
the feasibility of insulin gene replacement therapy, we
aimed to generate pancreatic beta cell insulin knockout mice
(MODY10) using CRISPR / Cas9. Subsequently, beta cells
carrying insulin gene defect will be transplanted into the kidney
capsule of diabetic subjects to develop MODY10 experimental
animal model of diabetes. For this purpose, two different insulin
gene therapy vectors with CMV and insulin promoters were
constructed. The gene expression profiles of these vectors were
first tested in 293T cells and then in NIT1 mouse pancreatic beta
cell lines. For the generation of the diabetic experimental animal
model, 8 weeks old male C57BL / 6J mice were intraperitoneally
injected with a single dose of 150 mg / kg Streptozotocin. After a
month of follow-up, continuous hyperglycemia was observed in
the subjects as a sign of destruction of pancreatic beta cells that
normally would express endogenous insulin. In the next step,
pancreatic beta cells with insulin gene deficiency will be generated
using CRISPR / Cas9 technique. TUBITAK(215S820)
Development of MODY10 diabetic experimental animal model using CRISPR/Cas9 technology for insulin gene transfer studies. Eksi Y, Altunbas HA and Sanlioglu S. ESGCT XXV Anniversary Congress. Human Gene Therapy. Volume:28 Issue:12 Pages: A48-A49 Meeting Abstract: P112. Berlin, Germany OCT 17-20, 2017
Made with Mobirise
Free Offline Site Software