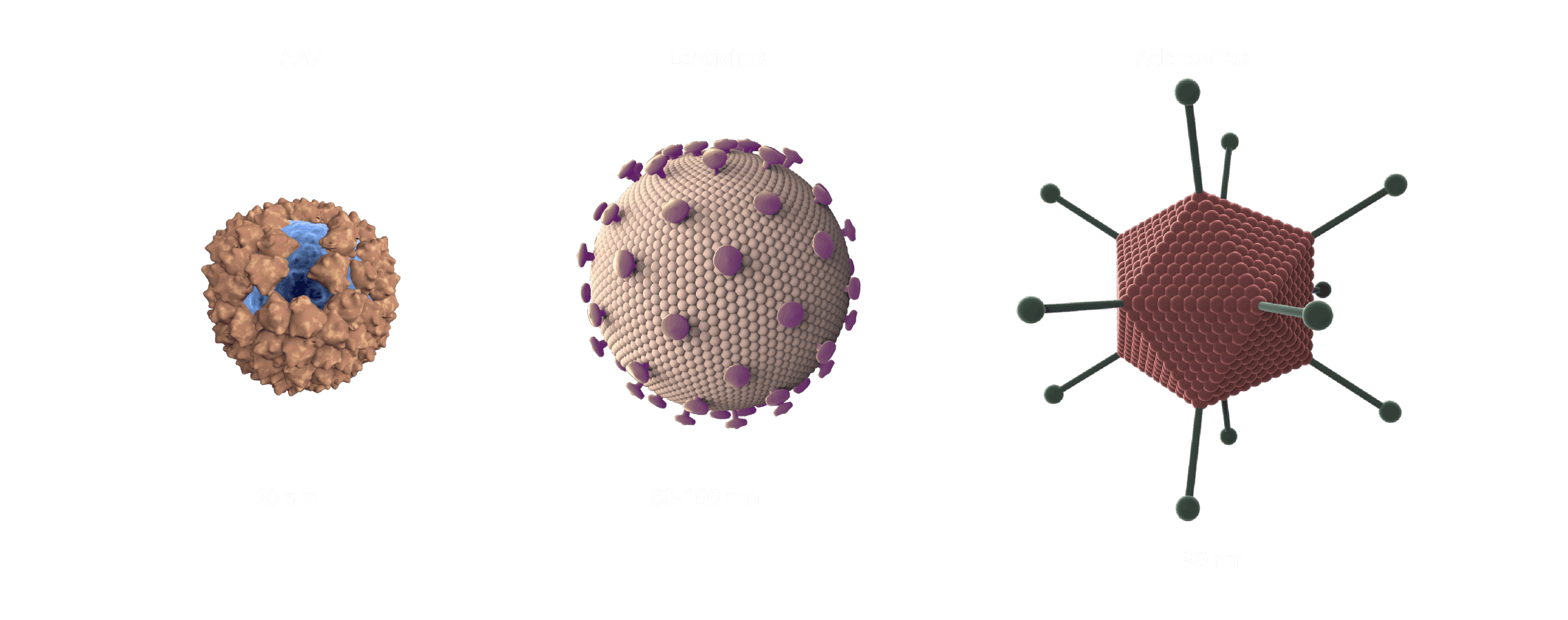
-1972, Friedmann and Roblin authored a paper in Science titled "Gene therapy for human genetic disease?" Rogers (1970) was cited for proposing that exogenous good DNA be used to replace the defective DNA in those who suffer from genetic defects
What is Gene Therapy?
Gene therapy is an experimental technique that uses genes to treat or prevent disease. In the future, this technique may allow doctors to treat a disorder by inserting a gene into a patient’s cells instead of using drugs or surgery. Researchers are testing several approaches to gene therapy, including:
-Replacing a mutated gene that causes disease with a healthy copy of the gene.
-Inactivating, or “knocking out,” a mutated gene that is functioning improperly.
-Introducing a new gene into the body to help fight a disease.
Although gene therapy is a promising treatment option for a number of diseases (including inherited disorders, some types of cancer, and certain viral infections), the technique remains risky and is still under study to make sure that it will be safe and effective. Gene therapy is currently being tested only for diseases that have no other cures. (Source: NIH).